News Story
UMD Researchers Develop Stable, Robust Li-ion Battery Chemistry

Although the flexible battery is cut, it continues to generate power.
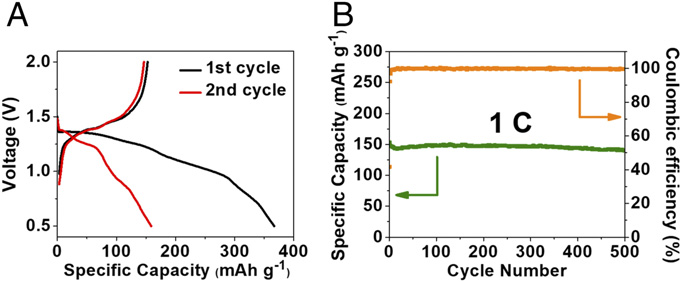
Lithium-ion (Li-ion) batteries power the lives of millions of people every day via mobile phones, laptop computers, iPods and even hybrid automobiles. Although reported to be volatile – as evidenced by Samsung’s Note 7 saga – this battery chemistry remains popular due to its high energy density, quick recharge rate and ease of transport. Indeed, researchers at the University of Maryland (UMD) – partnered with the John Hopkins Applied Physics Laboratory (APL) and the Army Research Lab (ARL) – have been working to develop an enhanced Li-ion battery, able to maintain its mechanical integrity under adverse conditions including bending, cutting and even liquid submersion.
This work is a follow-up to past UMD/ARL collaborations focusing on salt-water-based battery chemistry. Earlier this year, the team revealed the creation of a 4.0-volt aqueous Li-ion battery, based on a water-in-salt concept, capable of powering household electronics. The research was published in Joule this past September.
“UMD and ARL have explored several anode and cathode combinations that can be used within the stability window of our electrolyte,” said Chunsheng Wang, a professor in the UMD Department of Chemical and Biomolecular Engineering. “By collaborating with UMD & ARL, we are starting to transition this technology into novel battery architectures and demonstrate its practical true potential,” said Kostas Gerasopoulos, senior research scientist and principal investigator at APL.
In their most recent work, entitled, “Flexible Aqueous Li-ion Battery with High Energy and Power Densities,” the team inserted a salt water electrolyte in polyvinyl alcohol (PVA) solution to create a gel polymer electrolyte (GPE). This GPE was then combined with a material called lithium vanadium fluorophosphates, or LiVPO4F, which was utilized as both the anode and cathode, to create an incredibly stable and flexible battery. Over the last few years, LiVPO4F has frequently been used as the cathode in an organic electrolyte Li-ion, but this is the first time it’s been used in aqueous electrolyte symmetrical batteries.
“What makes LiVPO4F attractive for us is that it can be used as both anode and cathode within the stability window of the water-in-salt GPE, or alternatively, it can be matched with other high-voltage cathodes to achieve high energy density,” said Chongyin Yang, a UMD ChBE assistant research scientist and first author of the paper.
The most amazing attribute of this newfound technology is its robustness. “The cell can withstand cutting and continue to operate in an open cell condition without malfunction,” said Kang Xu, electrochemistry team leader and fellow at ARL. “To the best of our knowledge, this feature has not been previously reported for battery chemistries,” the team stated in their report. “The stability of the water-in-salt electrolyte in the air was also confirmed by monitoring the weight retention of water-in-salt GPE when exposing the electrolyte to air at room temperature for 20 days.” Follow this link to watch a video demonstration of the battery, provided by the John Hopkins APL.
This study was recently published in the journal Advanced Materials. For additional information, please cite the following:
Chongyin Yang, Xiao Ji, Xiulin Fan, Tao Gao, Liumin Suo, Fei Wang, Wei Sun, Ji Chen, Long Chen, Fudong Han, Ling Miao, Kang Xu, Konstantinos Gerasopoulos, Chunsheng Wang. Flexible Aqueous Li-Ion Battery with High Energy and Power Densities. Advanced Materials, 2017; DOI: 0.1002/adma.201701972.
Related Media:
Breakthrough Cuttable, Flexible, Submersible and Ballistic-Tested Lithium-ion Battery Offers New Paradigm of Safety and Performance - John Hopkins Applied Physics Lab, October, 2017
UMD Researchers Offer Solution to Volatile Battery Chemistry in Electronics – UMD/ChBE, September 2017
ChBE Researchers Offer New Salt-Water-Based Battery Chemistry – UMD/ChBE, June 2017
Published October 16, 2017