News Story
Ingestible Capsule Advances May Lead to Earlier Detection of Diseases
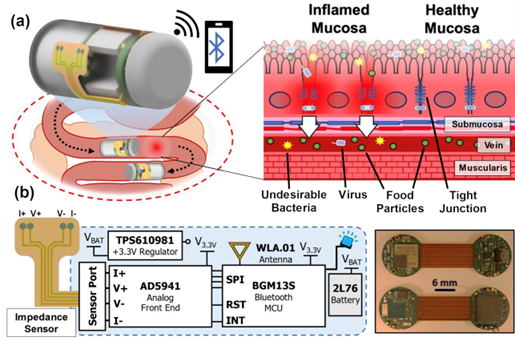
Figure 1 from the paper shows an overview schematic depicting the ingestible capsule monitoring bioimpedance in the small intestine. A diagram of ingestible bioimpedance sensing device traversing through GI tract and wirelessly transmitting impedance with illustration of tight junction dilation occurring during inflammatory processes in small intestinal mucosa is shown in (a). An electronics schematic of impedance sensing capsule and flex-rigid PCB is shown in (b).
University of Maryland-affiliated (UMD) researchers have made important progress in developing an ingestible capsule with non-invasive bioimpedance sensing that can identify the "leaky gut" precursors of many gastrointestinal (GI) tract conditions. A new invited paper detailing the research, "An ingestible bioimpedance sensing device for wireless monitoring of epithelial barriers," has been published in the Feb. 7, 2025 edition of the Nature journal, Microsystems and Nanoengineering.
"Leaky gut" conditions can predict chronic GI diseases - if they can be detected
The intestine’s internal mucous surfaces are lined with a thin protective layer of epithelial cells. These epithelia play a central role in regulating interactions between the mucosal immune system and the contents of the intestines, which include dietary antigens, a diverse intestinal microbiome, and pathogens. The epithelia establish a selectively permeable barrier that supports nutrient absorption and waste secretion while keeping out foreign material.
Spaces between epithelial cells are sealed by a complex network of protein interactions known as "tight junctions." Local inflammation contributes to changing how tight-junction proteins work. Poorly functioning, dilated tight junctions—"leaky gut"— allow undesirable bacteria into the intestines.
This condition can be an early indicator of the onset and progression of GI tract diseases such as Crohn's disease (CD) and ulcerative colitis (UC), and is implicated in Type 1 diabetes, celiac disease, multiple sclerosis, and a range of autoimmune disorders.
Currently, detecting leaky gut is difficult; a patient must undergo a biopsy or be subjected to an invasive diagnostic test like endoscopy. Non-invasive diagnostic solutions like wireless ingestible devices could provide a more comfortable and accessible method to detect leaky gut, enhancing the quality of life for the millions of people affected by gastrointestinal disorders.
"This new paper advances our previous research and demonstrates a novel, noise-resilient, Bluetooth-enabled wireless ingestible device," says Justin Stine, one of the lead authors. "We’ve come up with some creative solutions to address the challenges of in vivo diagnostics, like the capsule’s powerful miniaturized circuitry and coated sensor."
The paper was written by Brian Holt, Justin Stine, Luke Beardslee, Hammed Ayansola, Younggeon Jin, Pankaj Pasricha, and Reza Ghodssi. Holt, who earned an M.S. degree at UMD in 2024, is currently a cyber software engineer at Lockheed Martin. Stine is a UMD Assistant Research Scientist at the MATRIX Lab in California, Md. Beardslee is affiliated with the UMD Institute for Systems Research. Ayansola is a Ph.D. student and Jin is an assistant professor in the UMD Department of Animal and Avian Sciences. Pasricha, a physician and researcher specializing in gastroenterology and neurogastroenterology, is director of medicine to the Division of Gastroenterology and Hepatology and Department of Internal Medicine at the Mayo Clinic in Phoenix, Ariz. Ghodssi is a UMD professor with a joint appointment in the Department of Electrical and Computer Engineering and the Institute for Systems Research at the University of Maryland. He is Research Director for the MATRIX Lab and Director of the Microsensors and Actuators Lab. He was the graduate advisor to Holt and Stine.
Bioimpedance sensor innovations for ingestible capsules
Wireless sensing capsules for measuring gut biomarkers such as pH, temperature, and motility could continuously report numerical values for disease diagnosis or real-time feedback. In particular, bioimpedance sensing could measure tissue conductivity and characterize paracellular ion permeability—useful for detecting leaky gut. Effective monitoring of barrier integrity in the GI tract demands targeted impedance sensing, facilitated by a microfabricated sensor design and surface treatment to minimize noise. This must be integrated with miniaturized potentiated circuitry capable of wireless communication that can fit within a compact ingestible capsule.
To date, few ingestible devices in development explore GI tissue impedance beyond the esophagus. Challenges include integrating sensors and low-power electronics, interfacial impedance measurement noise, and difficult GI tract environmental conditions.
Here, the authors’ research addresses these challenges with a noise-resilient bioimpedance sensor coated with a conductive polymer film. The sensor integrates with ingestible capsule platforms and enables continuous characterization of mucosal ion permeability throughout the GI tract. This sensor can characterize changes in impedance caused by the dilation of tight junctions, an indicator of leaky gut.
"This groundbreaking work on an ingestible bioimpedance sensing device represents a significant advancement in the early detection and monitoring of gastrointestinal diseases. By enabling non-invasive, continuous measurement of intestinal mucosal "leakiness," this technology could revolutionize the diagnosis and management of many conditions," said Pasricha, one of the principal investigators. "Early detection of these diseases is crucial, as it can lead to more effective treatments, improved patient outcomes, and potentially prevent disease progression."
The authors have demonstrated the viability of their sensor design by validating it on treated and untreated excised porcine intestinal tissue. The device’s coated sensor successfully differentiated tissue that had been soaked in 1x phosphate-buffered saline (PBS) from untreated tissue. Through testing in a small animal trial of excised mice colonic tissue, the researchers proved the sensor can differentiate healthy and permeable tissues on a benchtop.
The resulting integrated capsule device can return a wireless readout of depth-specific impedance of GI tissues toward in vivo diagnostics. In addition, the sensor design process in this work may be adapted for monitoring other types of epithelial tissues.
"Dr. Reza Ghodssi and his colleagues have just published an exciting report on a "swallowable electronic pill" that could measure gut inflammation," said Frances Ligler, a Professor of Biomedical Engineering at Texas A&M University and Member of the National Academy of Engineering. "If the distribution of these microelectronic pills can be controlled inside the human gut, such measurements could be very important for diagnosis and treatment of diseases such as Crohn's and ulcerative colitis."
"This new work is a major step forward towards the use of non-invasive bioimpedance sensing as a diagnostic tool in ingestible technology and leaky gut identification," says Ghodssi. "We are grateful that our progress in ingestible capsule development continues to be recognized by Nature’s microsystems and nanotechnology community in this invited article."
Ingestible capsule development journey
In development since 2017, the ingestible capsule has been supported by a National Science Foundation (NSF) EAGER grant, the NSF ECCS Program, UMD TerrapinWorks, the Nanocenter FabLab, and the A. James Clark School of Engineering’s Mid-Career Doctoral Fellows Program. Innovations in the device’s development include:
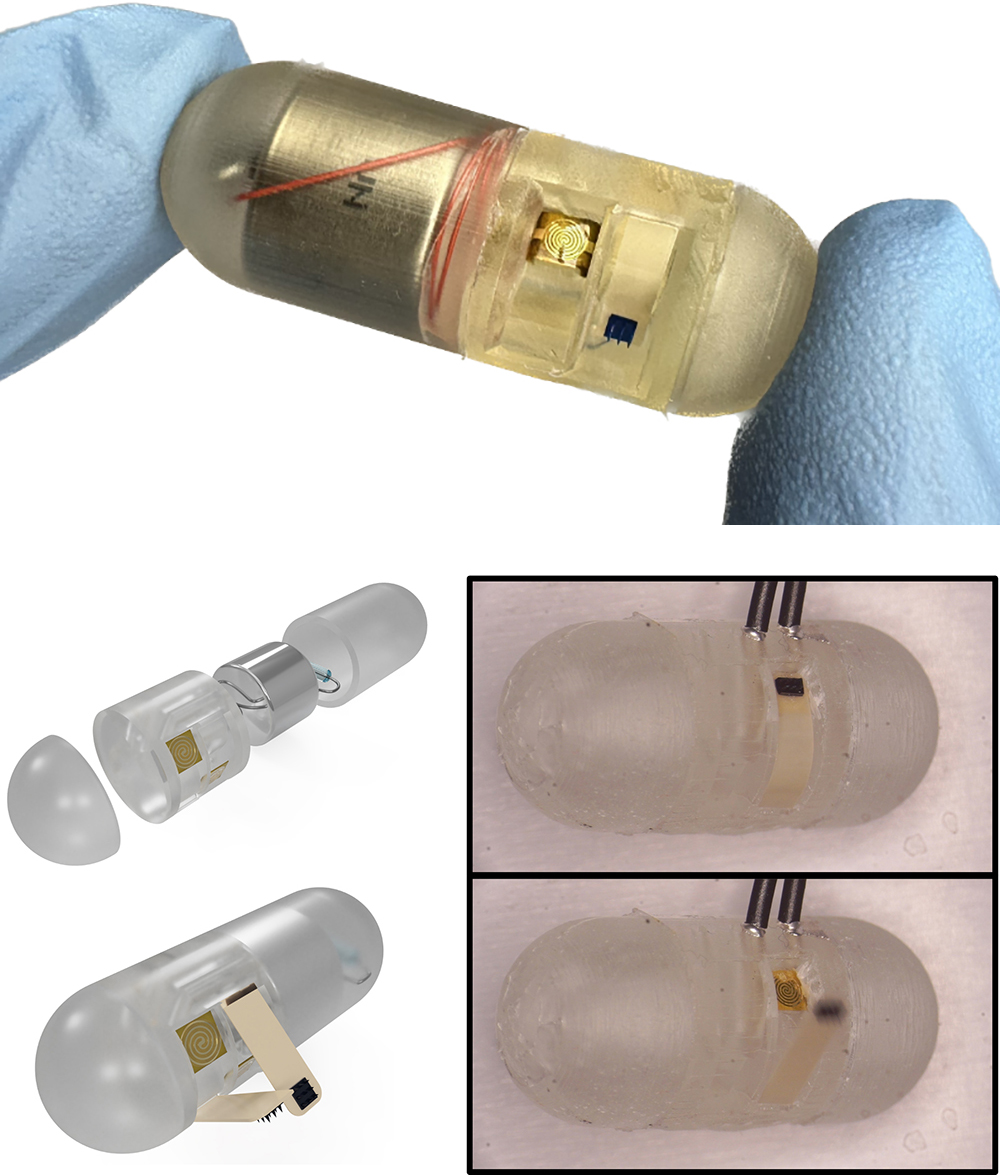
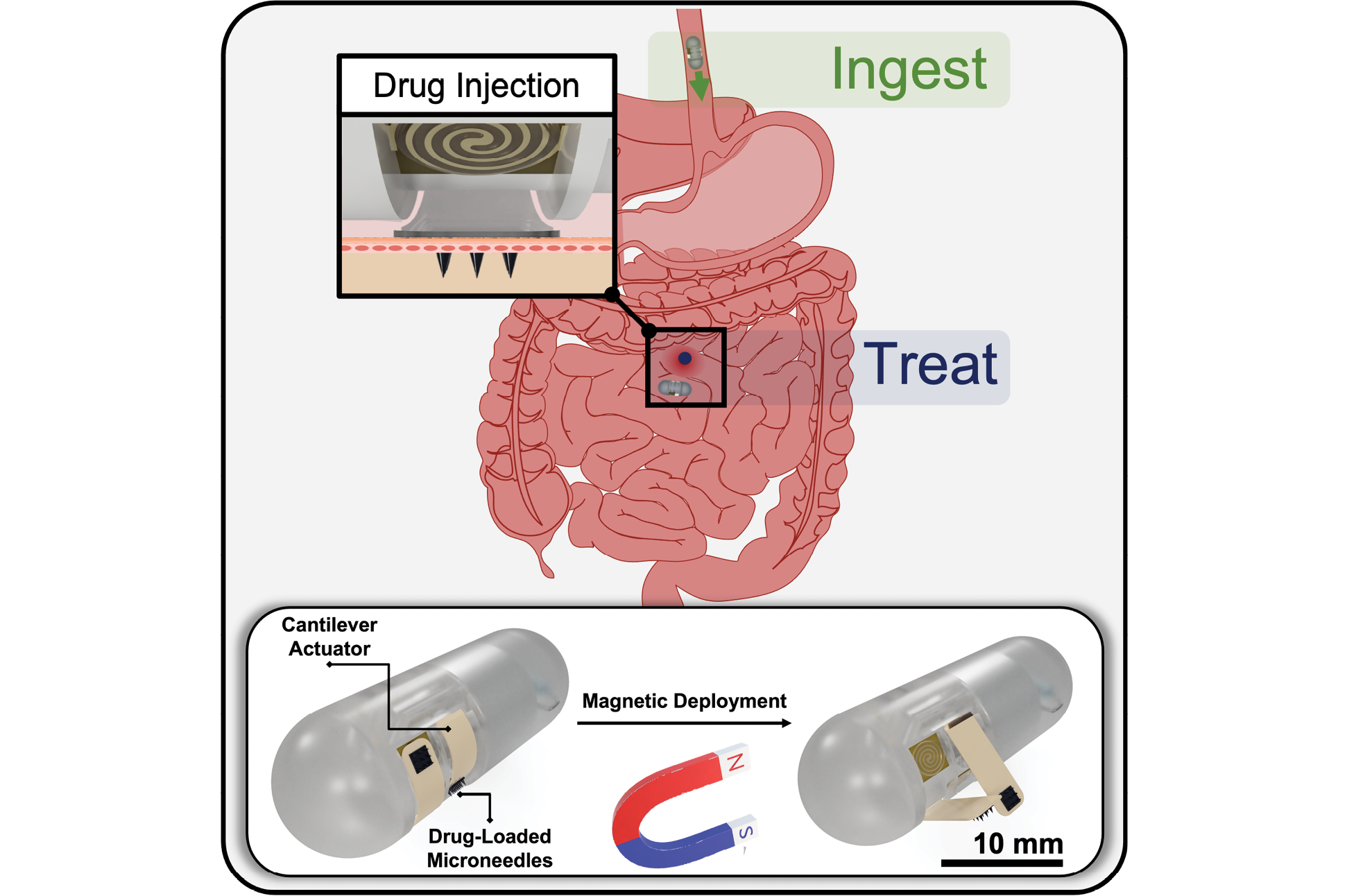
- New features on ingestible capsule will deliver targeted drugs to better treat IBD, Crohn’s disease (2024)
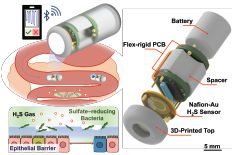
- Ingestible Capsule Technology Research on Front Cover of Advanced Healthcare Materials Journal (2024) and Gut Health Monitoring Gas Sensors Added to Ingestible Capsule Technology (2023)
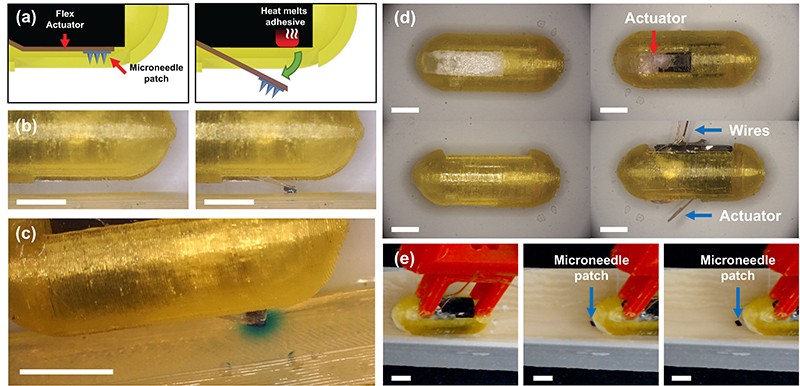
- New ‘FRRB’ packaging technology may solve an ingestible capsule challenge (2023)
- MSAL’s work on serotonin characterization and detection results in two journal covers (2023)
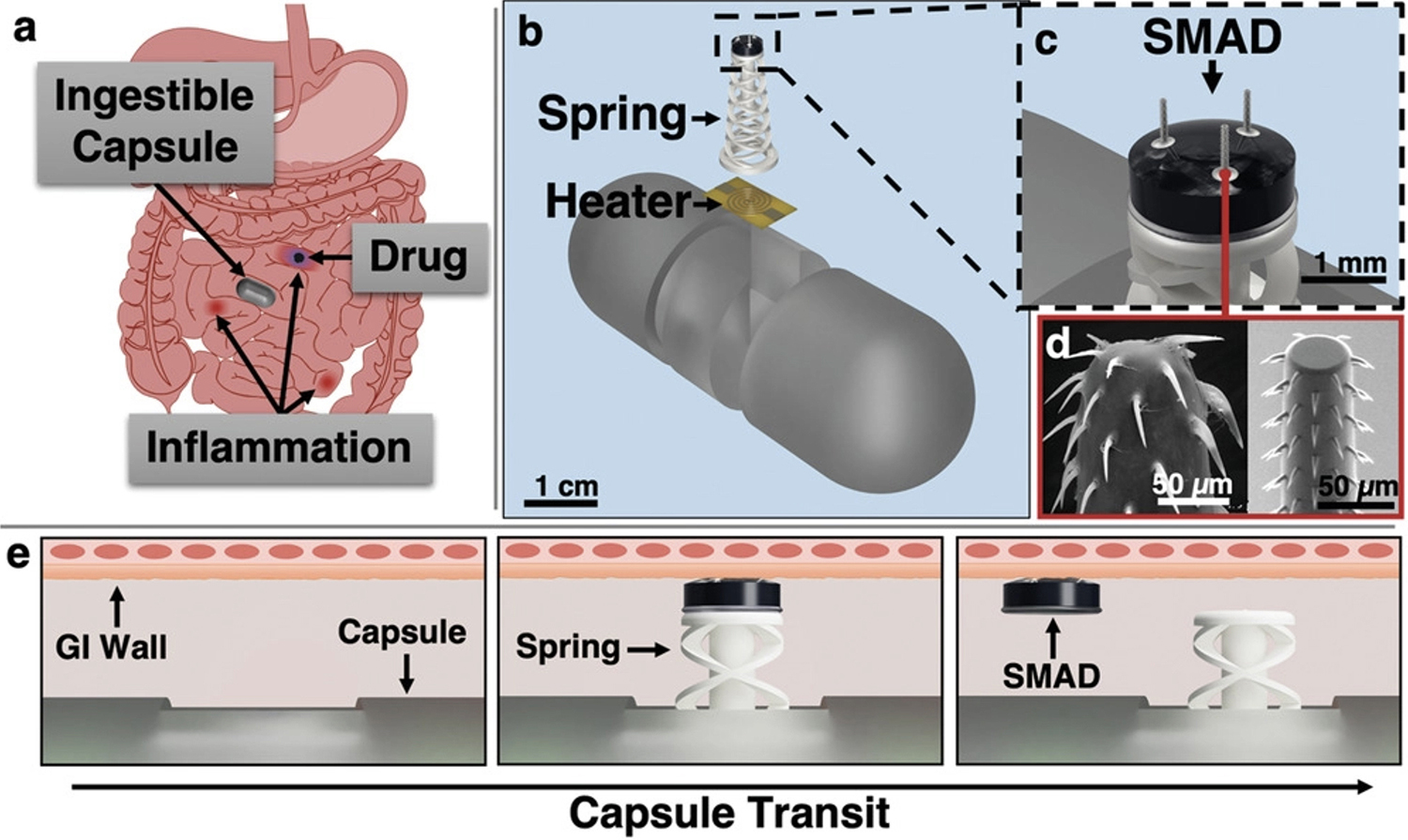
- Dropping an anchor for better GI tract disease treatment (2022)
- Fischell Fellowship advances visiting assistant professor’s work (2021)
- Ingestible device research advances, enters new phase (2020)
- Symptoms all in your head—or in your gut? Maybe a little of both (2020)
- NSF: Closed-Loop Sensing and Actuation for Gastrointestinal Capsule Systems (2020)
- Ghodssi, Bentley receive NSF EAGER grant to develop ingestible capsules for medical diagnosis (2017)
Published February 11, 2025