News Story
MSAL’s work on serotonin characterization and detection results in two journal covers
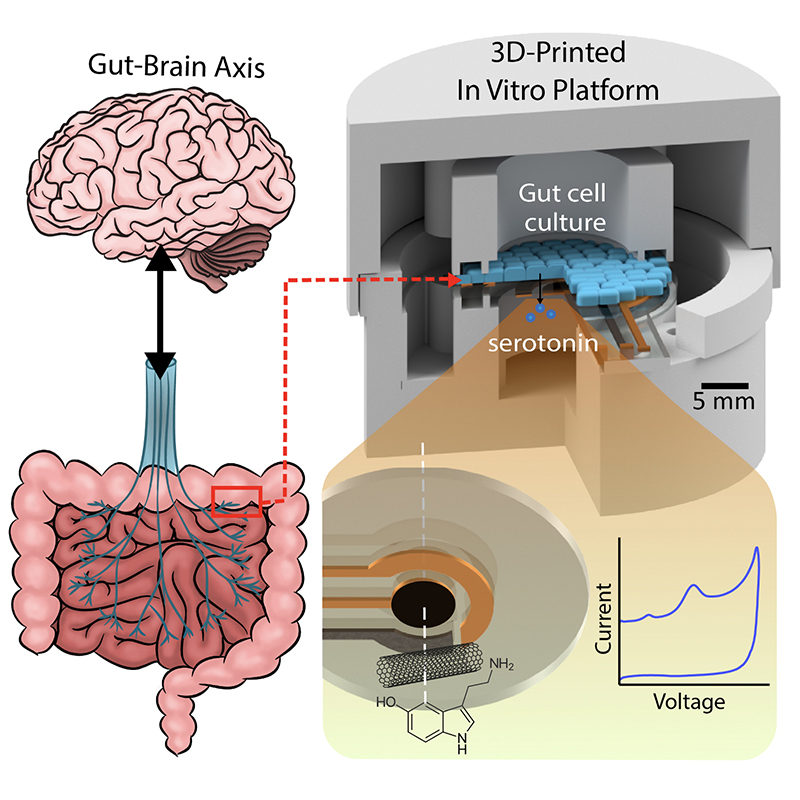
In recent years, researchers in the MEMS Sensors and Actuators Laboratory (MSAL) have been working on sensing, diagnosis and treatment solutions for medical problems that affect the brain and gastrointestinal (GI) tract, including the so-called “gut-microbiome-brain axis” (GBMA) through the vagus nerve.
“The interdisciplinary area of the Gut-Microbiome-Brain-Axis is a vital research frontier that provides a wide range of exciting research opportunities for our engineering graduate students and researchers,” Ghodssi says. “On this topic, Clark School students can work side by side with our colleagues in biology, physics, computer science, and the medical school to unravel the mystery of the communications between the human brain and the gut. Many scientific questions and medical causes could be investigated in this domain using better and more advanced engineering tools and methodologies.”
A major focus in MSAL has been sensing and measuring serotonin, a neurotransmitter involved in many biophysiological processes involving the brain and GI tract. Two new papers on serotonin characterization, detection and measurement by MSAL faculty, alumni and students were published as the cover articles of influential journals.
“Adsorption Kinetic Model Predicts and Improves Reliability of Electrochemical Serotonin Detection” was published in February 2023 in the MDPI journal Methods and Protocols. The paper was written by alum Ashley Chapin (BioE PhD 2022), current ECE PhD student Jinjing Han, and their advisor, Professor Reza Ghodssi (ECE/ISR).
“A Portable Electrochemical Sensing Platform for Serotonin Detection Based on Surface-Modified Carbon Fiber Microelectrode” was published in March 2023 in the Royal Society of Chemistry’s Analytical Methods. It was written by Jinjing Han, ECE PhD student Justin Stine, Ashley Chapin and Reza Ghodssi.
About serotonin
The neurotransmitter serotonin is secreted in the gut, where it activates local nerve fibers, creating signals which travel via the vagus nerve from the gut to the brain, underlying a variety of neurological and GI functions. In the brain, serotonin helps regulate behaviors such as sleep and learning, and plays a role in mood, social behavior, and emotional states such as fear, anxiety and depression. In the gut, serotonin is found in high levels, where it affects digestion, appetite, the movement of the involuntary muscles of the digestive system, visceral sensation, and nausea. Dysregulated serotonin has been implicated in sleep and mental health disorders, and a number of GI problems including diarrhea, constipation, Irritable Bowel Syndrome, and other chronic digestive diseases.
Medical researchers looking to better treat problems of the brain and the gut need to be able to accurately measure and characterize serotonin. Currently, such measurements require complex lab testing. But if serotonin concentrations within tissues or bodily fluids could be accurately quantified and correlated with biological processes in real time, the neurotransmitter’s levels could be adjusted more quickly. Quick and reliable doctor’s office testing or wearable/ingestible devices that a patient could monitor at home could bring real progress in treating and relieving symptoms of the underlying disease.
Advances in the two MSAL papers
Both papers are advances in the detection and measurement of serotonin. The Methods and Protocols paper by Chapin et. al., presents a model that tracks serotonin electrode adsorption and a resulting current output by combining Langmuir adsorption kinetic equations and adsorption-limited electrochemical equations. The Analytical Methods paper by Han, et.al., develops a simple, low-cost electrochemical sensing platform. It is a portable workstation with customized electrodes for serotonin detection in artificial biological fluids. The work has been successfully demonstrated on the benchtop and is in the process of being moved to the next step, demonstration in an in vivo animal model.
The adsorption kinetic model paper in Methods and Protocols
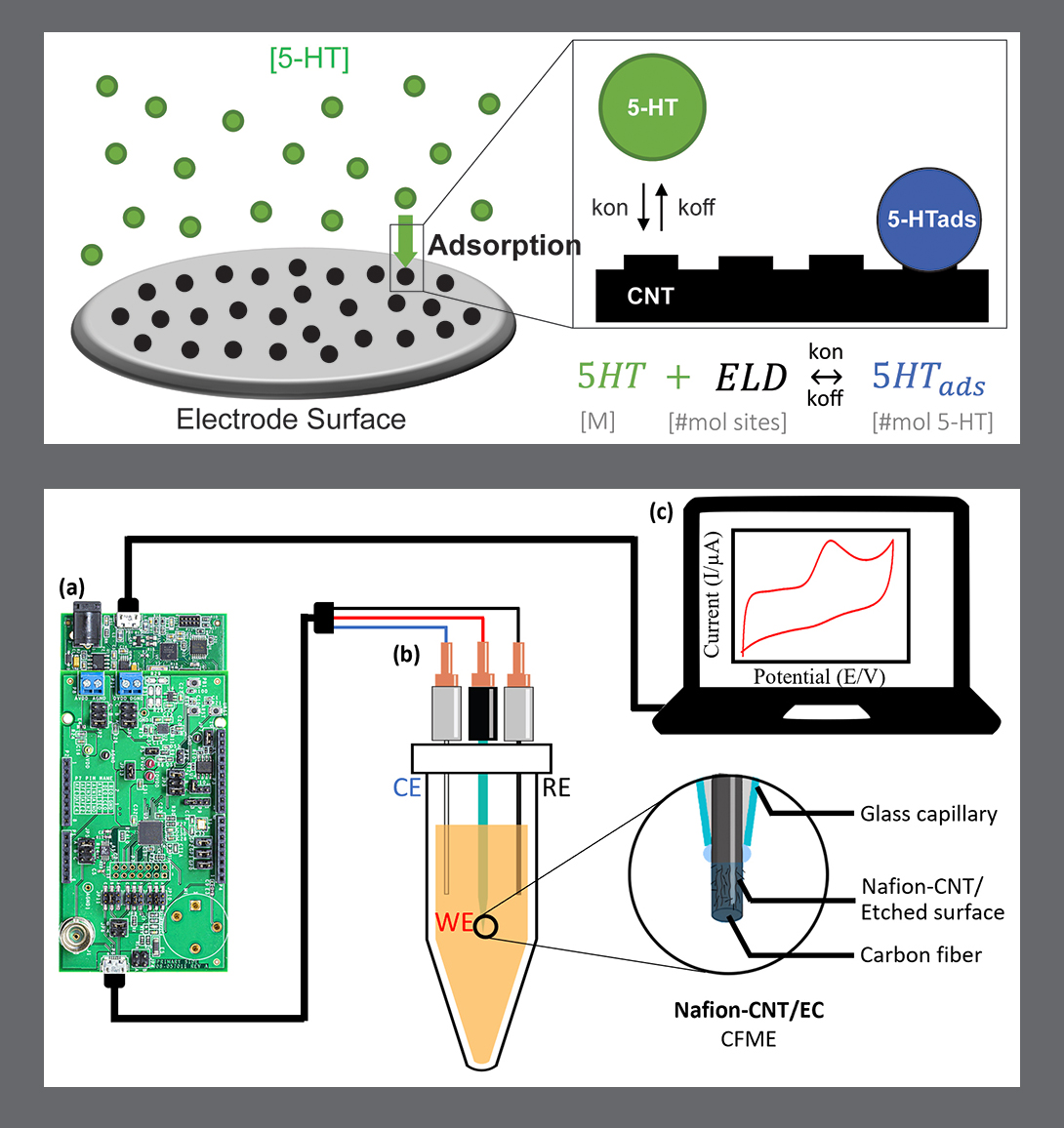
Electrochemical methods are commonly used to measure serotonin in real time. The serotonin molecules adsorb to an electroactive surface where they oxidize, producing a measurable electrical current signal. Unfortunately, the reliability of these methods often suffers for two reasons: the time-dependent nature of adsorption-limited serotonin detection, and the fouling of electrodes over repeated measurements. Mathematical characterization and modeling of adsorption-based electrochemical signal generation would improve reliability of serotonin measurement.
In this research, Chapin, Han and Ghodssi develop a simple but effective mathematical method to model serotonin electrochemical detection, based on experimentally-determined binding parameters with a given electrode. The method is derived from a combination of Langmuir and electrochemical adsorption equations.
The Langmuir isotherm model of adsorption commonly is used to study adsorption of molecules such as serotonin onto substrates—for example, electrodes. This type of model can quantify electrode sensitivity and linear range, and is less frequently used to determine fundamental binding parameters underlying electrochemical detection. Until this work, the Langmuir model has never been directly combined with adsorption-limited electrochemical equations that measure current in direct correlation with molecular concentration.
In this paper, the researchers demonstrate how an expanded adsorption model can predict and characterize the electrode detection of serotonin. The model effectively predicts both serotonin fouling and electrode responses over varying concentrations and measurement times. It can optimize and minimize the measurement time of serotonin secreted from a model enterochromaffin cell line, and can simplify and improve the characterization of serotonin detection at a selected electrode. The model could be useful when applied to other adsorption-limited electrochemical analytes and electrode types, and could improve application-specific modeling and optimization processes. It can act as a useful resource for many studies where molecular adsorption is the foundation of quantitative measurement.
“Although serotonin is an incredibly important neurotransmitter in both brain and GI physiology, fewer technological developments have been made to measure serotonin than other neurotransmitters (such as dopamine) because of serotonin fouling, which limits sensor lifetime,” says Chapin. “We envision that developing a framework to understand the processes that lead to fouling and variability in serotonin detection will help circumvent this ubiquitous limitation, ultimately leading to breakthrough technologies that aid in serotonin physiological research.”
The portable electrochemical sensing platform paper in Analytical Methods
In this research, Han, Stine, Chapin and Ghodssi have developed a simple, low-cost electrochemical sensing platform—a portable workstation with customized electrodes for serotonin detection that has demonstrated successfully in artificial biological fluids.
In the body, serotonin is produced and released into the blood, transported to the kidneys through systemic circulation, and then excreted into the urine. Urinary serotonin is a potential biomarker for serotonin imbalance-associated physiological conditions like serotonin syndrome, depression and carcinoid tumors.
Current analytical detection methods like liquid chromatography/tandem mass spectrometry and enzyme-linked immunosorbent assay can accurately detect serotonin levels, but their use requires a series of time-consuming sample preparation procedures, expensive instrumentation, and trained personnel.
Recent microelectronic advances in electrochemical analysis hardware have been changing the face of medical point-of-care testing. These new technologies are making simple, low-cost, and portable urinary serotonin testing possible, providing a solution for early diagnosis of disease and health self-monitoring.
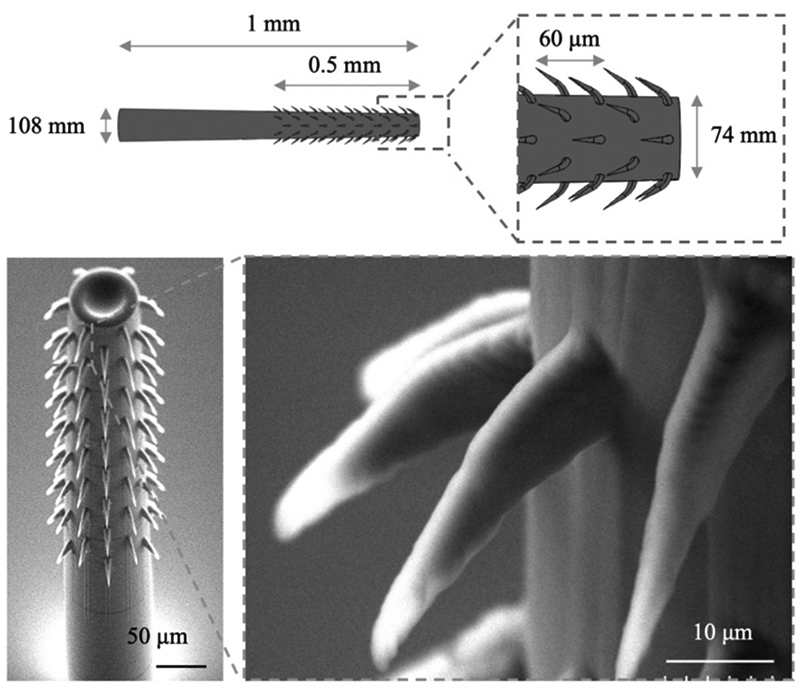
In this research, one of the biggest challenges the team faced involved the carbon-based electrodes used in sensing. These electrodes have been commonly used for electrochemical measurements because of their high electrical and thermal conductivities and corrosion resistance. However, bare carbon fiber electrodes have only a limited sensitivity for directly detecting serotonin at a physiologically relevant level. The electrodes need a custom coating to achieve fast, precise, selective, and sensitive results when sensing serotonin.
The research team developed carbon-based electrodes deposited with carbon nanotubes dispersed with Nafion. Nafion is a negatively charged, perfluorinated ion-exchange film that shows improved selectivity for serotonin, over negatively charged interference molecules like uric acid or ascorbic acid during electrostatic interactions. Carbon nanotubes—cylindrical graphite sheets with nanometer dimensions—provide a large surface-to-volume ratio and surface area. Their unique structures provide increased sensitivity, electron transfer kinetics, and electrocatalytic activity with minimal surface fouling.
The system can accurately measure serotonin concentrations in artificial urine samples, with nearly full recovery rates—an outstanding performance that confirms the feasibility of serotonin detection for future point-of-care analysis in actual biological samples and the potential for use in future self-monitoring devices. The platform will next be tested in vivo in an animal model. It also will be further miniaturized and optimized so it can be used for continuous self-monitoring of serotonin levels as a wearable or implantable device.
“Our platform has the potential to offer a cost-effective, rapid, and reliable electrochemical sensing method that could be utilized for early diagnosis in a point-of-care setting,” Han says. “By leveraging these novel technologies, we hope to significantly advance the field of electrochemical sensing and improve diagnostic outcomes for patients.”
More information
Learn about the $1 million NSF grant to investigate the complex effects of intestinal serotonin on both gut and brain health currently funding this work. The four-year project, which began in 2019 and concludes this fall, is providing a more realistic picture of the complex gut-microbiome-brain axis system. Professor Ghodssi is the principal investigator.
Read a summary of three previous important papers that resulted from this funding in 2020.
View a 2020 video about a model platform to study the gut-brain axis, featuring Ashley Chapin. The University of Maryland created the video for the Universitas 21 “Three-Minute Thesis” competition.
Published March 6, 2023